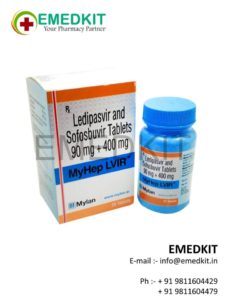
MyHep LVIR
MyHep LVIR tablet is a combination of two antiviral medicines- Ledipasvir and sofosbuvir. The combination medicines are found in a fixed dose of Ledipasvir (90mg) and Sofosbuvir (400mg) in a single form. These tablets are manufactured by MyLan Laboratories Ltd. (A global speciality and generic pharmaceuticals company) and you can Enquiry MyHep LVIR online from anywhere in the world especially USA, Russia, Romania, Peru and China. These tablets are known to work by decreasing the amount of Hepatitis C in the body and with time reducing the virus from the blood. MyHep LVIR tablets are used to treat the chronic Hepatitis C virus infection patients.
INDICATIONS AND USAGE
MyHep LVIR is indicated for the treatment of choronic hepatitis C (CHC) genotype 1 infection in adults. MyHepLVIR is two-drug fixed-dose combination product that contained 90 mg of ledipasvir and 400 mg of sofosbuvir in a single tablet.
DOSAGES AND ADMINISTRATION
• Recommended Dosage for Adults only
• The recommended dosage of MyHepLVIR is one tablet to be taken orally
• To be consumed once daily with or without food
• Consume the dosage as prescribed by the doctor ensuring to consume at a fixed time. The tabets can be found worldwide in a plastic bottle containing 28 tablets.
Duration of treatment
Although, generally this medication follows a 12 week plan to completely cure the patient. The relapse rates are affected by baseline host and viral factors and differ between treatment durations for certain subgroups.
the recommended LVIR treatment durations for treatment-naive and treatment-experienced patients and those with and without cirrhosis are given below:
Recommended Treatment Duration for MyHepLVIR in Patients with CHC Genotype 1:-
• Treatment-naive with or without cirrhosis 12 weeks
• Treatment-experienced without cirrhosis 12 weeks
• Treatment-experienced with cirrhosis 24 weeks
• MyHep LVIR for 8 weeks can be considered in treatment- naive patients without cirrhosis who have pre-treatment HCV RNA less than 6 million IU/ mL .
• Treatment-experienced patients who have failed treatment with either peginterferon alfa+ribavirin or an HCV protease inhibitor + peginterferon alfa + ribavirin
DOSAGES FORMS AND STERENGTHS
Each tablet contains 90 mg ledipasvir and 400 mg sofosbuvir. MyHep LVIR is available as Green colored, oval shaped, film coated tablet debossed with on one side and plain on other side of the tablet.
Side Effects
Apart from its intended effect, Myhep Lvir Tablet may cause some unwanted effects too. In such cases, you must seek medical attention immediately.
• Fatigue
• Nausea
• Headache
• Insomnia (difficulty in sleeping)
• Anemia.
The above mentioned is not an exhaustive list of side effects. Make sure to inform your doctor if you experience any adverse reaction to the medication.
Contradictions
Serious Symptomatic Bradycardia When Coadministered with Amiodarone
Postmarketing cases of symptomatic bradycardia, as well as fetal cardiac arrest and cases requiring pacemaker intervention, have been reported when amiodarone is coadministered with Ledipasvir + Sofosbuvir. Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease may be at increased risk for symptomatic bradycardia with coadministration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this effect is unknown.
ADVERSE REACTIONS
Clinical Trials Experience
The most common adverse reactions (>=10%) were fatigue and headache Nausea, Diarrhea, Insomnia in subjects treated with 8, 12, or 24 weeks of Ledipasvir + Sofodbuvir.
ALL HEPATITIS C MEDICAT
Clinical Trials Experience
The most common adverse reactions (>=10%) were fatigue and headache Nausea, Diarrhea, Insomnia in subjects treated with 8, 12, or 24 weeks of Ledipasvir + Sofodbuvir.
ALL HEPATITIS C MEDICATIONS CONTAINING LEDIPASVIR (90mg) & SOFOSBUVIR (400mg) ARE MANUFACTURED UNDER A LICENSE FROM GILEAD SCIENCES IRELAND UC.




Reviews
There are no reviews yet.