Product Description
What is dolutegravir?
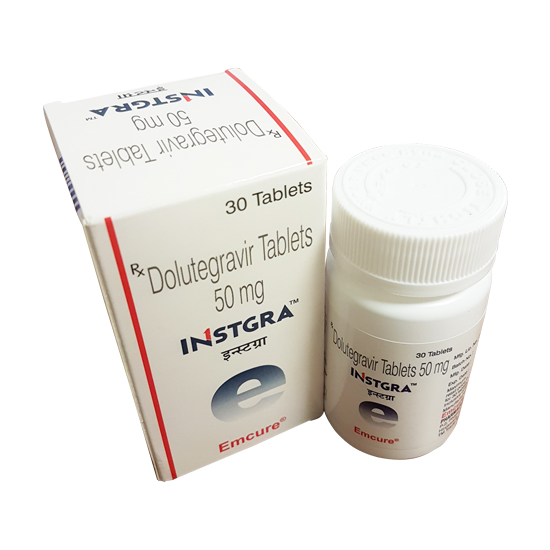
Dolutegravir is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV infection in adults and children 12 years of age and older and weighing at least 88 pounds (40 kilograms). Dolutegravir is always used in combination with other HIV medicines.
Dolutegravir belongs to a class (group) of HIV drugs called integrase inhibitors. Integrase inhibitors block an HIV enzyme called integrase. (An enzyme is a protein that starts or increases the speed of a chemical reaction.) By blocking integrase, integrase inhibitors prevent HIV from multiplying and can reduce the amount of HIV in the body. HIV medicines can’t cure HIV/AIDS, but taking a combination of HIV medicines (called an HIV regimen) every day helps people with HIV live longer, healthier lives. HIV medicines also reduce the risk of HIV transmission.
What should I tell my health care provider before taking dolutegravir?
Before taking dolutegravir, tell your health care provider:
If you are allergic to dolutegravir or any other medicines.
If you have had or currently have liver problems, including hepatitis B virus (HBV) infection or hepatitis c virus (HCV) infection.
If you have any other medical conditions.
If you are pregnant or plan to become pregnant. Whether dolutegravir can harm an unborn baby is unknown. Dolutegravir should be used during pregnancy only if the potential benefits outweigh the risks. Talk to your health care provider about possible risks with taking dolutegravir when pregnant.
If you are breastfeeding or plan to breastfeed. Do not breastfeed if you are infected with HIV or are taking dolutegravir.
If you are using hormone-based birth control (such as pills, injections, or implants).
About other prescription and nonprescription medicines, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Dolutegravir may affect the way other medicines or products work, and other medicines or products may affect how dolutegravir works. Taking dolutegravir together with certain medicines or products may cause serious side effects. Do not take dolutegravir if you take dofetilide. Taking dolutegravir and dofetilide can cause side effects that may be life threatening.
How should I take dolutegravir?
Dolutegravir (brand name: Tivicay) comes in tablet form. Each tablet contains 50 mg dolutegravir.
Take dolutegravir according to your health care provider’s instructions.
Take dolutegravir by mouth, with or without food.
Always take dolutegravir in combination with other HIV medicines.
If you take too much dolutegravir, contact your health care provider or local poison control center (1-800-222-1222) right away, or go to the nearest hospital emergency room.For more information on how to take dolutegravir, see the FDA drug label from DailyMed. (DailyMed is a federal website that includes the most recent drug labels submitted to FDA.)
What should I do if I forget a dose?
If you miss a dose of dolutegravir, take the missed dose as soon as you remember it. But if it is within 4 hours of your next dose, skip the missed dose and just take your next dose at the regular time. Do not take two doses at the same time to make up for a missed dose.
What side effects can dolutegravir cause?
Dolutegravir may cause side effects. Most side effects from dolutegravir are manageable, but a few can be serious. Serious side effects of dolutegravir include allergic reactions and liver problems. (See the WARNING above.)Other possible side effects of dolutegravir include:
Changes in liver test results. People with a history of HBV or HCV may have an increased risk of developing new or worsening changes in certain liver test results during treatment with dolutegravir.
Changes in body fat (including gain or loss of fat).
Immune reconstitution inflammatory syndrome (IRIS), a condition that sometimes occurs when the immune system begins to recover after treatment with an HIV medicine. As the immune system gets stronger, it may have an increased response to a previously hidden infection.
Trouble sleeping.
Tiredness.
Headache.
Tell your health care provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of dolutegravir. To learn more about possible side effects of dolutegravir, read the drug label or package insert or talk to your health care provider or pharmacist.The AIDSinfo fact sheet on HIV Medicines and Side Effects also includes information that may apply to dolutegravir.
How should dolutegravir be stored?
Store dolutegravir at room temperature, 68°F to 77°F (20°C to 25°C).
Do not use dolutegravir if the original seal over the bottle opening is broken or missing.
Throw away dolutegravir that is no longer needed or expired (out of date). Follow FDA guidelines on how to safely dispose of unused medicine.
Keep dolutegravir and all medicines out of reach of children.
Where can I find more information about dolutegravir?
More information about dolutegravir is available:
The dolutegravir drug label, from DailyMed. The Patient Counseling Information section of the label includes information for people taking dolutegravir.
Dolutegravir-related research studies, from the AIDSinfo database of



Reviews
There are no reviews yet.